This information was developed by the Publications Committee of the American Society for Gastrointestinal Endoscopy (ASGE). For more information about ASGE, visit www.asge.org.
This information is intended only to provide general guidance. It does not provide definitive medical advice. It is important that you consult your doctor about your specific condition.

The Benefits of Endoscopy
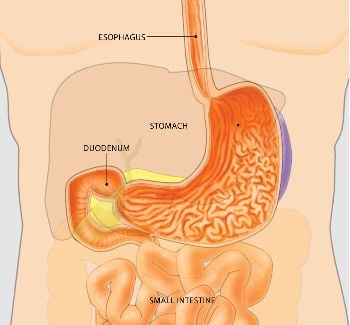
Endoscopy involves the use of flexible tubes, known as endoscopes, to provide a close-up, color television view of the inside of the digestive tract. Upper endoscopes are passed through the mouth to visualize the esophagus (food pipe), stomach, and duodenum (first portion of the small intestine), while lower endoscopes (colonoscopes) are passed through the rectum to view the colon or large intestine. Other special endoscopes allow physicians to view portions of the pancreas, liver and gallbladder as well.
Endoscopy has been a major advance in the treatment of gastrointestinal diseases. For example, the use of endoscopes allows the detection of ulcers, cancers, polyps and
sites of internal bleeding. Through endoscopy, tissue samples (biopsies) may be obtained, areas of blockage can be opened, and active bleeding can be stopped. Polyps in the colon can be removed, which has been shown to prevent colon cancer.
Endoscopy is easily carried out on an outpatient basis and is very well tolerated by patients. The technique of endoscopy is extremely safe, with very low rates of complications, when performed by a properly trained endoscopist, such as members of the American Society for Gastrointestinal Endoscopy (ASGE).
The Characteristics of an Endoscope
An endoscope consists of a flexible tube, which is passed into the digestive tract to provide a video image, and a control section, which allows the endoscopist to maneuver the tip of the flexible tube in a precise manner. Within the tube are the electronics necessary to obtain the video image, cables that allow control of the flexible tip, and channels that permit the passage of devices to sample tissue, stop bleeding, or remove polyps. The endoscope is a complex but durable instrument and is safe for use in thousands of procedures.
Effectiveness of the Reprocessing Guidelines
The dissemination and implementation of the guidelines for endoscope reprocessing (cleaning and disinfecting) outlined here have resulted in a remarkable safety record for endoscopy. Based on medical literature, the Technology Committee of the ASGE estimates that the chance that a serious infection could be transmitted by endoscopy is only about 1 in 1.8 million. Given the multiple benefits of endoscopy, it is no wonder that the number of procedures performed grows each year and that endoscopy is a mainstay of digestive disease treatment plans and health maintenance strategies. Endoscope manufacturers are continually improving the design of endoscopes to ensure patient safety.
Quality Assurance and Training
Any facility in which gastrointestinal endoscopy is performed must have an effective quality assurance program in place to ensure that endoscopes are reprocessed properly. Quality assurance programs for endoscopy must include the supervision, training, and annual competency review of all staff involved in the process, systems that assure availability of appropriate equipment and supplies at all times, and strict procedures for reporting possible problems.
Availability of Reprocessing Guidelines
The ASGE guidelines for infection control during gastrointestinal endoscopy provide the latest techniques and step-by-step directions on the proper procedure for cleaning and disinfecting endoscopes. These are distributed to all members of ASGE and are regularly reviewed and updated. They are also easily accessed on the ASGE Web site (www.asge.org) or by calling or writing ASGE.
How the Preparation of an Endoscope for Each Procedure Ensures Patient Safety
In all areas of medicine and surgery, complex medical devices are generally not discarded after use in one patient but rather are reused in subsequent patients.
This practice is very safe, provided that the devices are properly prepared, or reprocessed, prior to each procedure, so as to eliminate any risk that an infection could be transmitted from one patient to another.
Prior to the performance of a procedure, an endoscope must be carefully cleaned and disinfected according to guidelines published by the American Society for Gastrointestinal Endoscopy, which have been endorsed by every major medical association dealing with endoscopy and infection control. The steps involved in cleaning and disinfecting an endoscope are as follows:
Mechanical cleaning. The operating channels and external portions of the endoscope are washed thoroughly, wiped with special liquids that contain enzymes, and brushed with special cleaning instruments. Studies have shown that these steps alone can eliminate potentially harmful viruses and other microbes from an endoscope. However, much more is done before the endoscope is considered ready for use.
Leakage testing. The endoscope is tested to be sure that there are no leaks in its internal operating channels. This not only ensures peak performance of the endoscope, but also allows immediate detection of internal defects that could be a potential focus of infection within the device. Despite its complex electronics, an entire endoscope can be submersed completely in liquid so that leakage testing can be carried out.
Use of chemical disinfectants. Next, the endoscope is soaked continuously for an appropriate time period with one of several approved liquid chemicals that destroy microorganisms that can cause infections in humans, including the AIDS virus, hepatitis viruses, and potentially harmful bacteria. There are a variety of chemical disinfectants used to achieve high-level disinfection. This process eliminates virtually all microbial life except for some inactivate dormant organisms known as spores. However, spores are uncommonly found in endoscopes and, even if present, are not harmful to humans. Although most high-level disinfectants are also sterilants (which kill all spores), this requires a much longer exposure time, and has not been shown to be necessary. The human mouth, small intestine, colon and rectum contain millions of non-harmful bacteria. Therefore, as soon as the endoscope touches the internal surface of a patient, it is not sterile. The goal of a “sterile” endoscope from the beginning to the end of a procedure is not achievable. Therefore, the goal of reprocessing is to eliminate from the endoscope any potentially harmful microbes. This goal can be achieved with high-level disinfectant chemicals and by following standard reprocessing guidelines.
Rinsing and drying. After exposure to the chemical disinfectant, the endoscope channels are flushed with sterile water followed by alcohol and then air-dried to eliminate any moisture that could be a site of bacterial growth from the environment. The endoscope is then stored on a specialized hanger to keep it dry and free of contamination.

Important Reminder: This information is intended only to provide general guidance. It does not provide definitive medical advice. It is very important that you consult your doctor about your specific condition.
Since its founding in 1941, the American Society for Gastrointestinal Endoscopy (ASGE) has been dedicated to advancing patient care and digestive health by promoting excellence in gastrointestinal endoscopy. ASGE, with more than 11,000 members worldwide, promotes the highest standards for endoscopic training and practice, fosters endoscopic research, and is the foremost resource for endoscopic education.
This patient education brochure was developed by the Publications Committee of the American Society for Gastrointestinal Endoscopy. This information is the opinion of and provided by the American Society for Gastrointestinal Endoscopy.
American Society for Gastrointestinal Endoscopy www.asge.org and www.screen4coloncancer.org
Copyright ©2010. American Society for Gastrointestinal Endoscopy. All rights reserved. This information may not be reproduced without express written permission by ASGE. For permission requests, please contact the ASGE Communications Department at 630-673-0600.